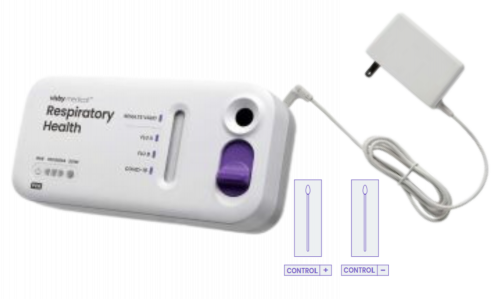
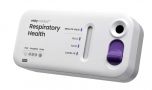
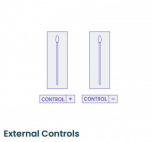
Visby Medical Respiratory Health - Starter Kit
Product Discontinued! Please purchase individual units below:
-
Visby Respiratory PCR Health Tests - COVID-19/FLU A&B (cliawaived.com)
-
Visby Respiratory Health Tests - Controls (cliawaived.com)
-
Visby Health Test Power Cord (cliawaived.com)
The Visby Medical Respiratory Health Test is a single-use (disposable), fully integrated, rapid, automated RT-PCR in vitro diagnostic test intended for the simultaneous qualitative detection and differentiation of SARS-CoV-2, influenza A, and influenza B viral RNA in healthcare provider-collected nasopharyngeal and anterior nasal swab specimens, and healthcare provider-instructed self-collected anterior nasal swab specimens (collected on site) from individuals with signs and symptoms of respiratory tract infection consistent with COVID-19.
Ground Shipping only, please call 858.481.5031 for expedited shipping options.
NOTE: Additional power cords should be ordered if you plan to run multiple tests at the same time.
Limited to 1 Starter Pack Per Ship Location!
Clinical Performance -
· Covid PPA 97.4%, NPA 98.8%
· Flu A PPA 97.9% NPA 99.3%
· Flu B PPA 96.9% NPA 100%
CPT Code Map
Visby Medical Respiratory Health Test:
- Easy to use: One swab, three targets: COVID-19, influenza A and influenza B
- Affordable: No maintenance or service contracts
- Scalable: Easy to run multiple test devices at the same time
The Visby Medical Respiratory Health Test is a single-use (disposable), fully integrated, fast, compact device containing a reverse transcription polymerase chain reaction (RT-PCR) based assay for qualitative detection of influenza A, influenza B, and/or SARS-CoV-2 viral RNA in upper respiratory tract specimens. The device automatically performs all steps required to complete lysis, reverse transcription (RT), PCR amplification, and detection.