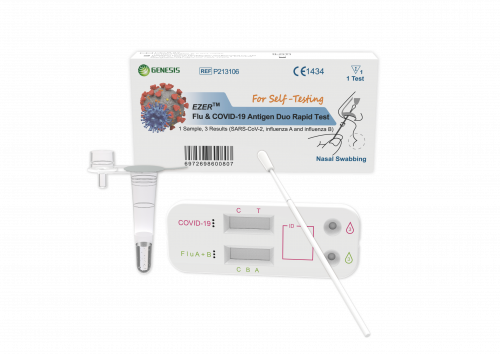
EZER™ Flu & COVID-19 Antigen Duo Rapid Test
Minimum order of 10,000 tests.
A rapid test for the detection of influenza A, influenza Band SARS-CoV-2 antigens in nasal specimens.
- For self-testing use.
- Use test only one time
- Testing by adult only or under adult supervision.
- Carefully read the instructions before performing the test. Failure to follow the instructions may result in inaccurate test results.
Approved for self-testing and for sale in the following countries ONLY (Not for sale in the USA):
Austria, Belgium, Bulgaria, Croatia, Republic of Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, Netherlands, Poland, Portugal, Romania, Slovakia, Slovenia, Spain and Sweden.
EZER™ Flu & COVID-19 Antigen Duo Rapid Test is a lateral flow immunoassay intended for the in vitro rapid, simultaneous qualitative detection and differentiation of nucleocapsid antigen from SARS-CoV-2, influenza A and influenza B from nasal swab specimens obtained from individuals, who are suspected of respiratory viral infection, within the first three days of onset of symptoms. Clinical signs and symptoms of respiratory viral infection due to SARS-CoV-2 and influenza can be similar.
This test is for laymen with self-collected nasal (nares) swab samples from individuals aged 14 years or older, or adult collected nasal swab samples from individuals aged 2 years or older.
Results are for the simultaneous identification of nucleocapsid antigens of SARSCoV-2, influenza A and influenza B, but does not differentiate between SARS-CoV and SARS-CoV-2 viruses.
These viral antigens are generally detectable in nasal swab samples during the acute phase of infection. Positive results do not rule out bacterial infection or coinfection with other viruses. The agent detected may not be the definite cause of the disease. Positive result is advised to consult With a medical practitioner, and follow local authorities· recommendations. and not take any decision of medical relevance without first consulting their medical practitioner.
Negative results should be considered in the context of an individual's recent exposures. history and the presence of clinical signs and symptoms consistent with COVID-19, and confirmed with a molecular assay, if necessary, for patient management. Negative influenza A and B test results should be treated as presumptive. It is recommended these results be confirmed an influenza A and B molecular assay. Negative results do not preclude influenza virus infection and should not be used as the sole basis for treatment or other management decisions.
User should contact medical practitioner if symptoms or other infection suspicion persist.
EZER Flu & COVID-19 Antigen Duo test is not for sale in the USA.