Flowflex COVID -19 Antigen Home Test (25 test pack)
FlowFlex Home Covid test kit (25 test pack)
ITEM DISCONTINUED
We are sorry to inform you that this product has been discontinued.
We are offering below options as alternatives:
-
Quidel Quickvue At Home OTC COVID-19 Tests (25 dipsticks per box)
-
Flowflex COVID -19 Antigen Home Test (50 individual test packs)
-
GenBody COVID-19 Rapid Antigen Tests (25 tests per kit)
-
INDICAID™ COVID-19 Rapid Antigen Test Kit (cliawaived.com)
All EUA authorized COVID-19 products are non-returnable with no exceptions unless damaged in transit, product is recalled for manufacturer erformacne issues or deemed defective by the manufacturer. Buyer should beware that EUA authorized products could be revoked by FDA and removed from authorization list. Products that are revoked are non-returnable and non-refundable. Note: Manufacturer recalls are returnable for replacement product only. No Cash refund will be given for manufacturer-initiated recall.
|
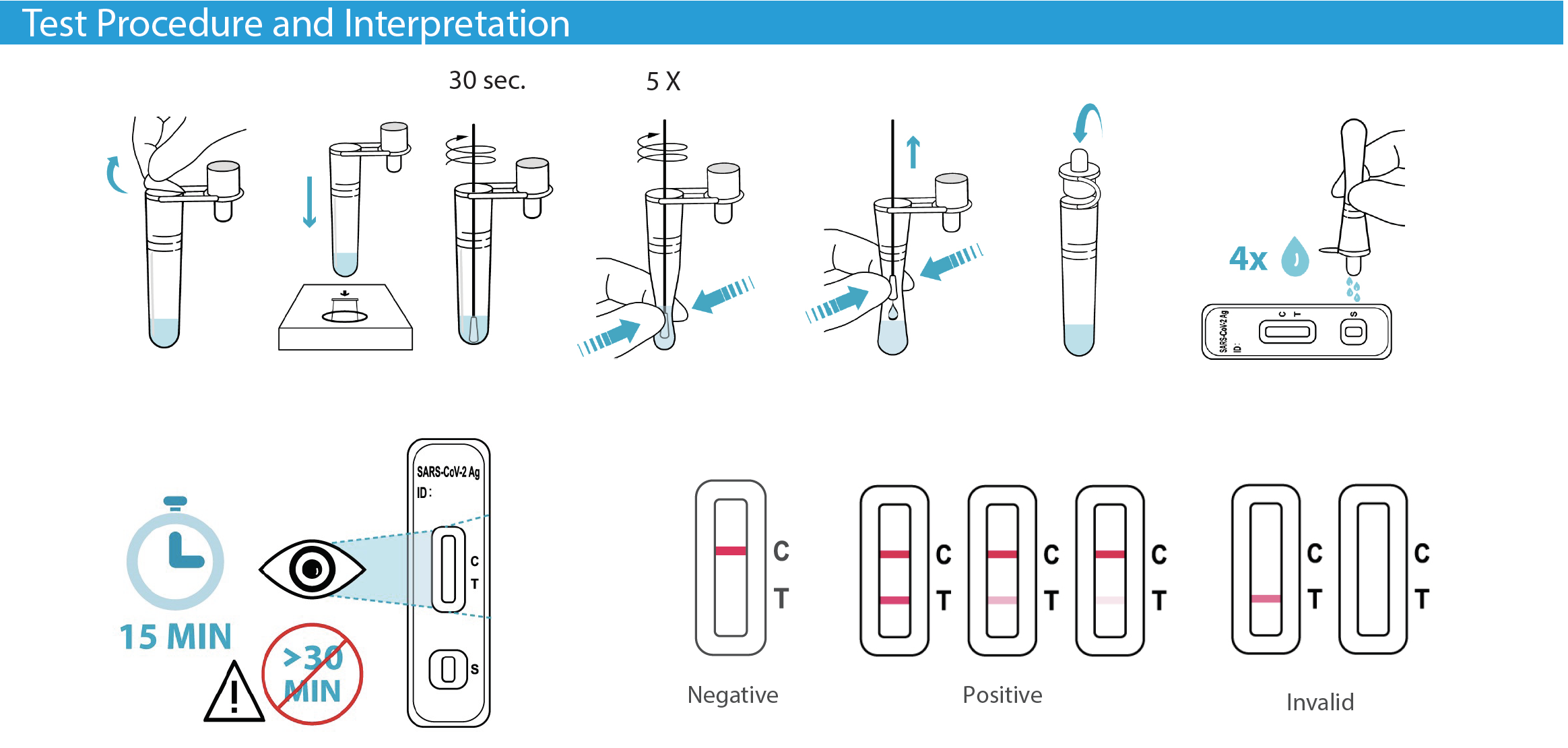
• Anterior nasal swab specimens • Results in 15 minutes • 12 Months shelf life • Store between 36 to 86° F |
• Sample self-collection ages 14 and older • Sample collection by an adult in children ages 2 to 13 • Excellent performance when compared to an FDA authorized molecular SARS-CoV-2 test. |

The Flowflex COVID-19 Antigen Home Test was compared to an FDA authorized molecular SARS-CoV-2 test. The Flowflex COVID-19 Antigen Home Test
correctly identified 93% of positive specimens and 100% of negative specimens.

|
• Test Cassette(s) • Package Insert |
• Extraction Buffer Tube(s) • Nasal Swab(s) |
• External Tube Holder - Package of 25 tests |
A rapid test for the detection of SARS-CoV-2 antigens in anterior nasal specimens directly from individuals within 7 days of symptom onset or without symptoms or other epidemiological reasons to suspect COVID-19 infection.
Qualitative detection of the nucleocapsid protein antigen from SARS-CoV-2 in anterior nasal swab specimens directly from individuals within 7 days of symptom onset or without symptoms or other epidemiological reasons to suspect COVID-19 infection.
This test is authorized for non-prescription home use with self-collected anterior nasal swab specimens directly from individuals aged 14 years and older or with adult-collected anterior nasal samples directly from individuals aged 2 years or older.
“In the USA, this product has not been FDA cleared or approved, but has been authorized by FDA under an EUA. This product has been authorized only for the detection of proteins from SARS- CoV-2, not for any other viruses or pathogens. The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated, or authorization is revoked sooner.