Quidel Quickvue At Home OTC COVID-19 Tests (25 dipsticks per box)
Out of Stock! Please see alternative below:
(25 Tests per Box)
This item is packaged as 1 bulk box that contains 25 single dipsticks in a tube. Not designed for retail pharmacy resale.
The QuickVue At-Home OTC COVID-19 Test is intended for the qualitative detection of the nucleocapsid protein antigen from SARS-CoV-2 from individuals with or without symptoms or other epidemiological reasons to suspect COVID-19 when tested twice over two or three days with at least 24 hours and no more than 36 hours between tests. This test is authorized for nonprescription home use with self-collected (unobserved) direct anterior nasal (NS) swab specimens from individuals aged 14 years and older or with adult-collected anterior NS samples from individuals aged 2 years or older.
The QuickVue At-Home OTC COVID-19 Test does not differentiate between SARS-CoV and SARS-CoV-2.
Made in USA COVID-19 Tests - (Meets requirements for "The Buy American Act")
All EUA authorized COVID-19 products are non-returnable with no exceptions unless damaged in transit, product is recalled for manufacturer erformacne issues or deemed defective by the manufacturer. Buyer should beware that EUA authorized products could be revoked by FDA and removed from authorization list. Products that are revoked are non-returnable and non-refundable. Note: Manufacturer recalls are returnable for replacement product only. No Cash refund will be given for manufacturer-initiated recall.
The QuickVue At-Home OTC COVID-19 Test is intended for the qualitative detection of the nucleocapsid protein antigen from SARS-CoV-2 from individuals with or without symptoms or other epidemiological reasons to suspect COVID-19 when tested twice over two or three days with at least 24 hours and no more than 36 hours between tests. This test is authorized for nonprescription home use with self-collected (unobserved) direct anterior nasal (NS) swab specimens from individuals aged 14 years and older or with adult-collected anterior NS samples from individuals aged 2 years or older.
The QuickVue At-Home OTC COVID-19 Test does not differentiate between SARS-CoV and SARS-CoV-2.
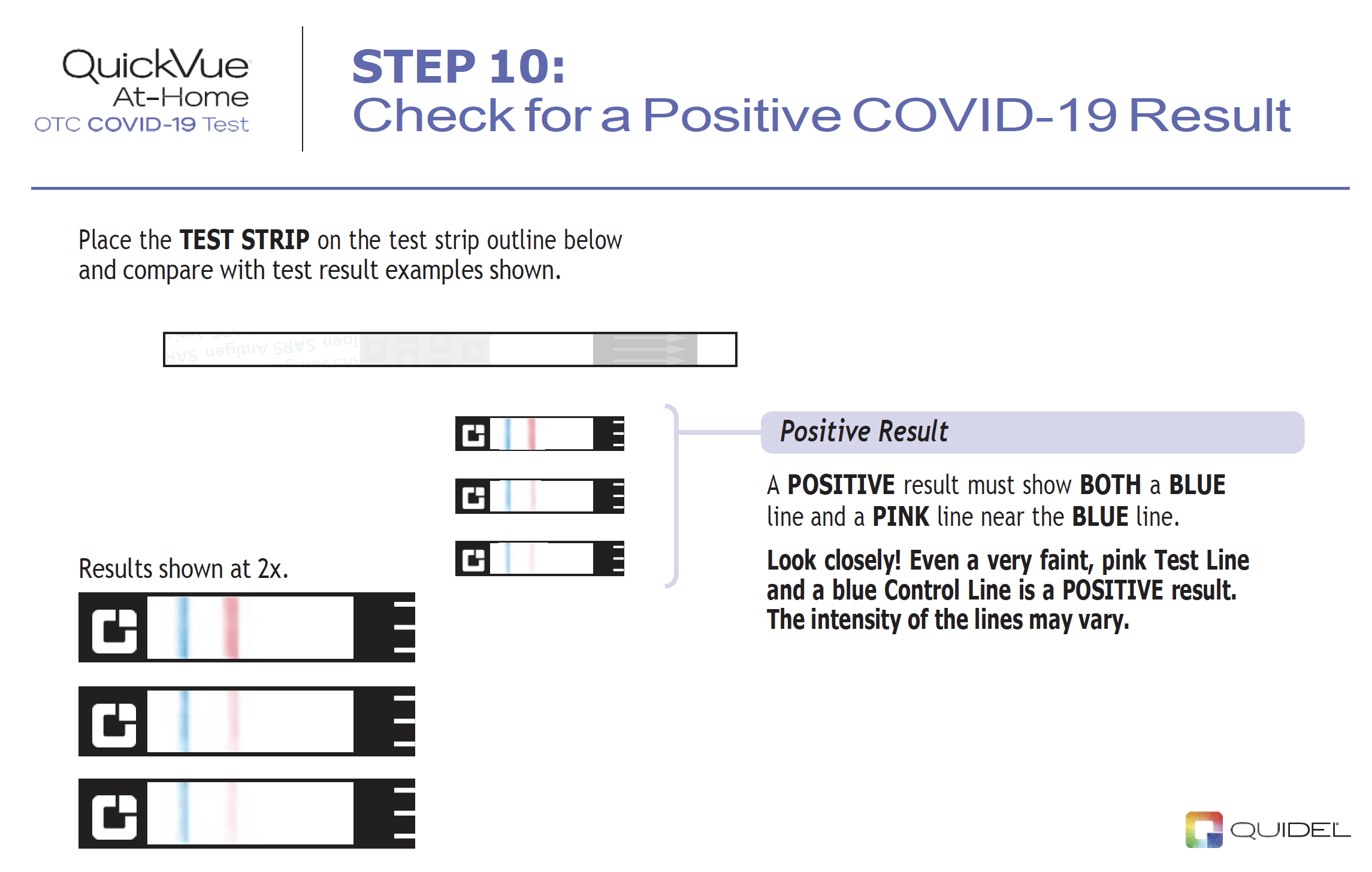
Results are for the identification of SARS-CoV-2 nucleocapsid protein antigen. Antigen is generally detectable in anterior nasal specimens during the acute phase of infection. Positive results indicate the presence of viral antigens, but clinical correlation with past medical history and other diagnostic information is necessary to determine infection status. Positive results do not rule out bacterial infection or co-infection with other viruses and the agent detected may not be the definite cause of disease. Individuals who test positive with the QuickVue At-Home OTC COVID-19 Test should self-isolate and seek follow-up care with their physician or healthcare provider as additional testing may be necessary.
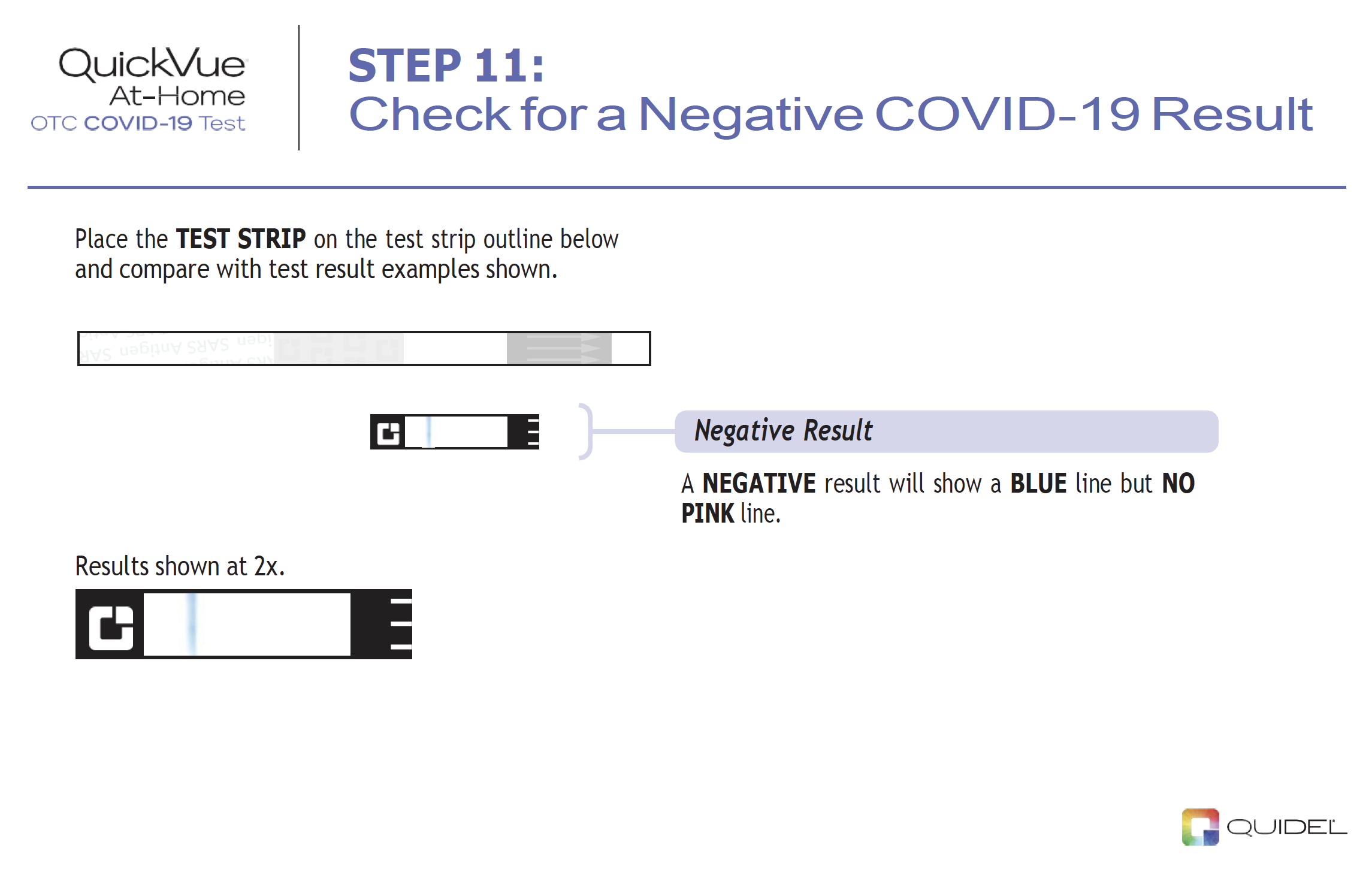
Negative results should be treated as presumptive, do not rule out SARS-CoV-2 infection and should not be used as the sole basis for treatment or patient management decisions, including infection control decisions. Negative results should be considered in the context of an individual’s recent exposures, history and the presence of clinical signs and symptoms consistent with COVID-19, and confirmed with a molecular assay, if necessary, for patient management. For serial testing programs, additional confirmatory testing with a molecular test for negative results may be necessary, if there is a high likelihood of SARS-CoV-2 infection, such as in an individual with as a close contact with COVID-19 or with suspected exposure to COVID-19 or in communities with high prevalence of infection. Additional confirmatory testing with a molecular test for positive results may also be necessary, if there is a low likelihood of SARS-CoV-2 infection, such as in individuals without known exposures to SARS-CoV-2 or residing in communities with low prevalence of infection.
Individuals who test negative and continue to experience COVID-19 like symptoms of fever, cough and/or shortness of breath may still have SARS-CoV-2 infection and should seek follow up care with their physician or healthcare provider.
Individuals should provide all results obtained with this product to their healthcare provider for public health reporting. All healthcare providers will report all test results they receive from individuals who use the authorized product to relevant public health authorities in accordance with local, state, and federal requirements using appropriate LOINC and SNOMED codes, as defined by the Laboratory In Vitro Diagnostics (LIVD) Test Code Mapping for SARS-CoV-2 Tests provided by CDC.
The QuickVue At-Home OTC COVID-19 Test is authorized for non-prescription self-use and/or, as applicable for an adult lay user testing another person aged 2 years or older in a non-laboratory setting. The QuickVue At-Home OTC COVID-19 Test is only for use under the Food and Drug Administration’s Emergency Use Authorization.
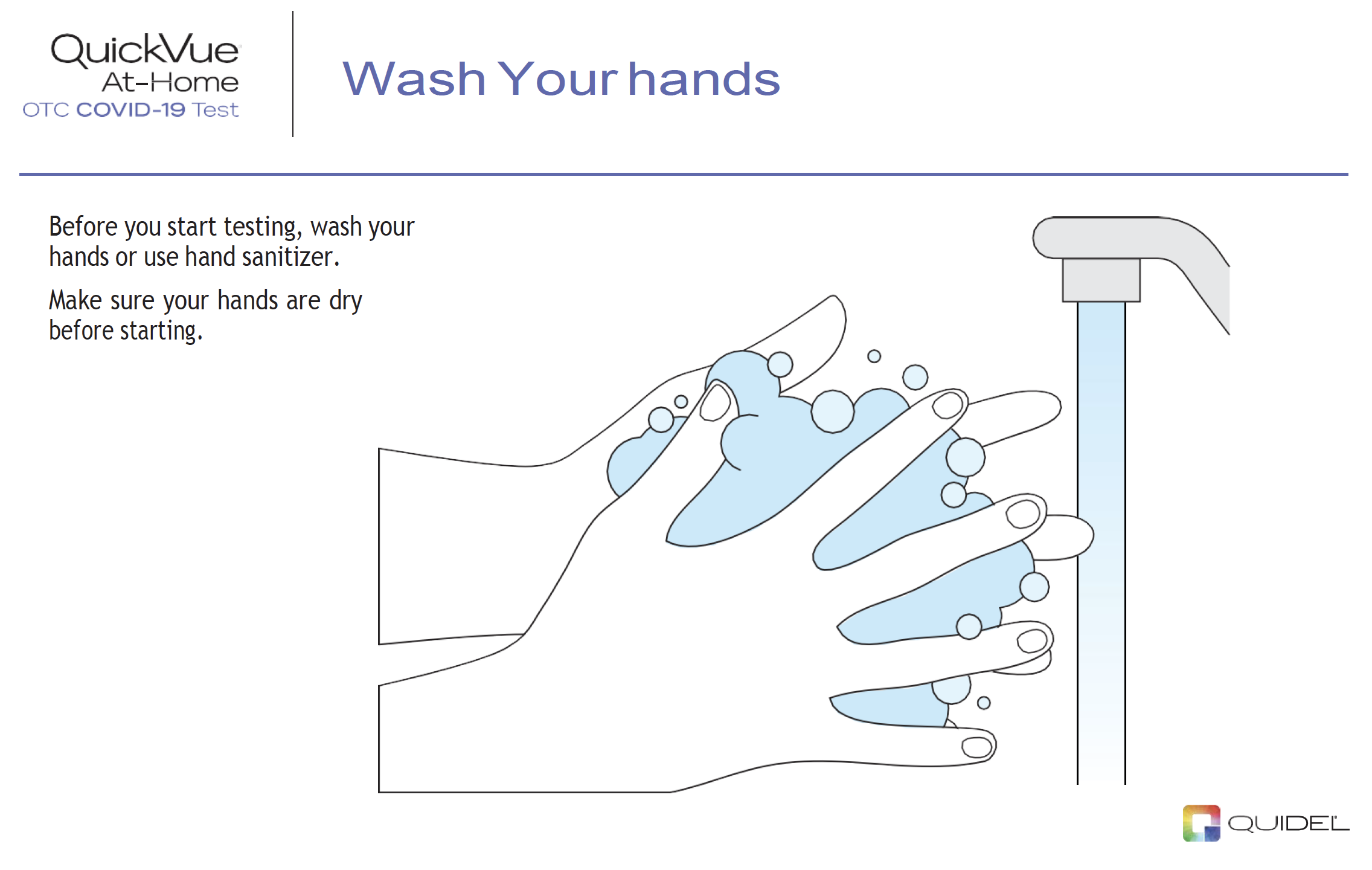 |
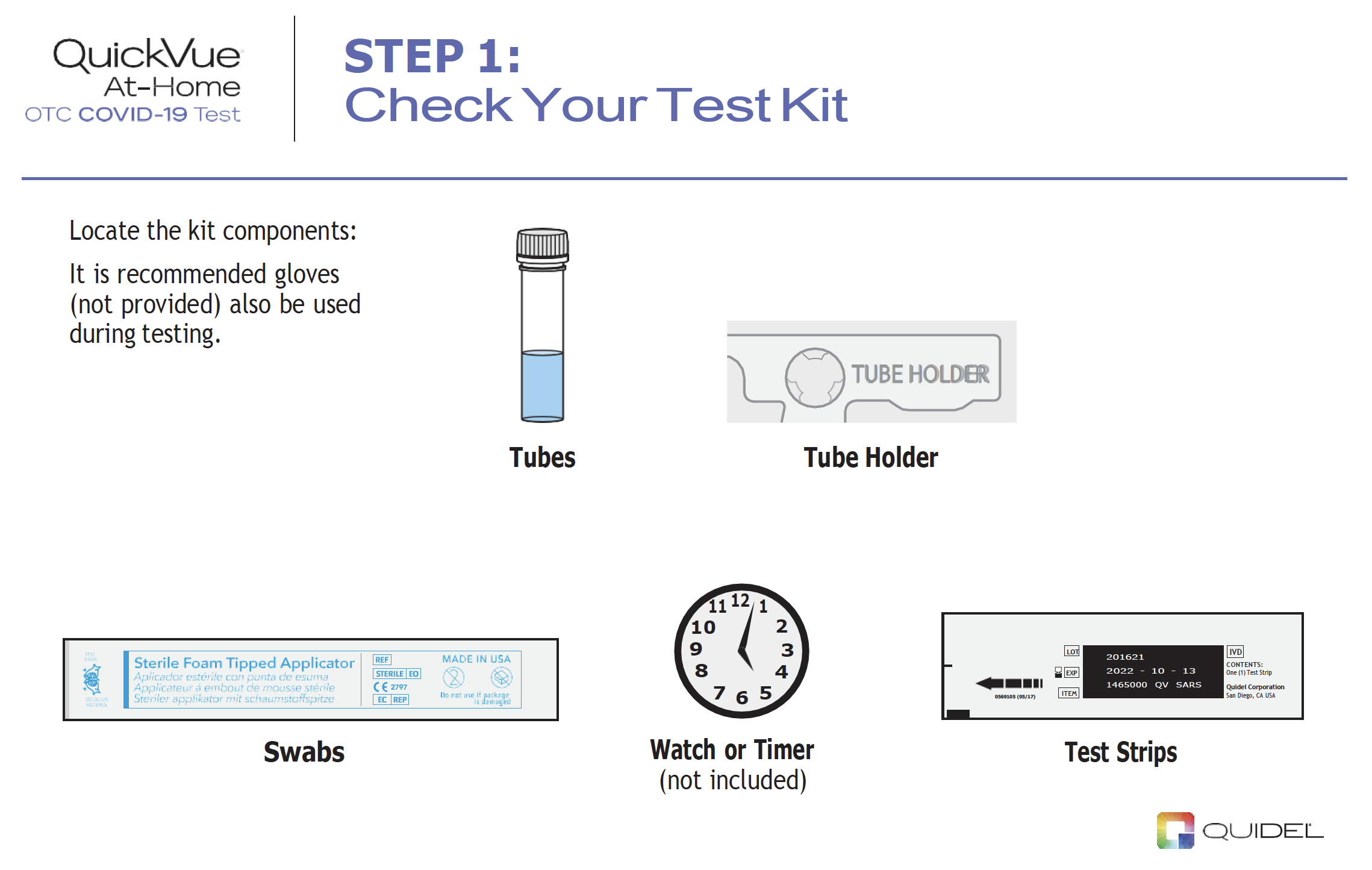 |
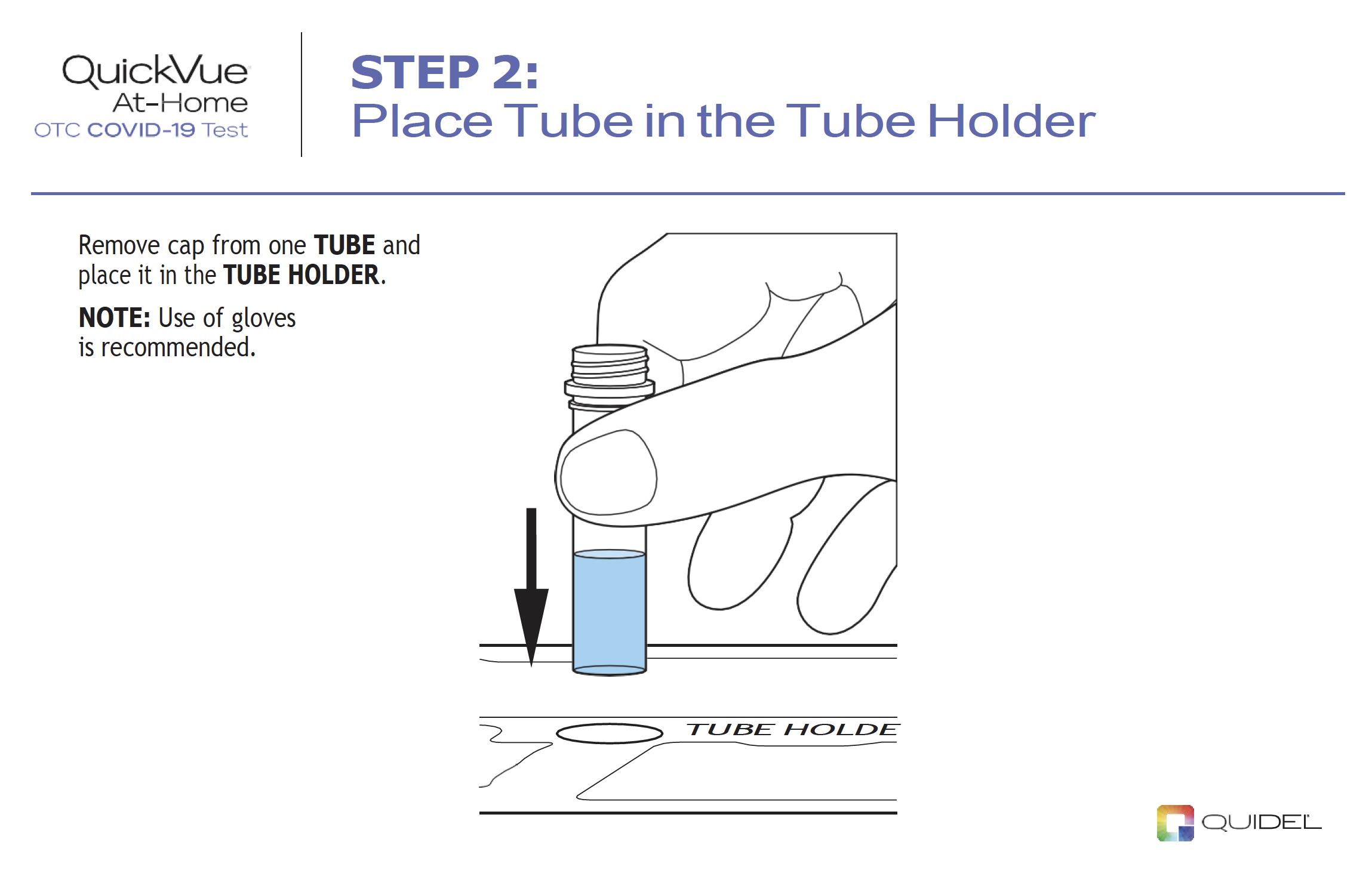 |
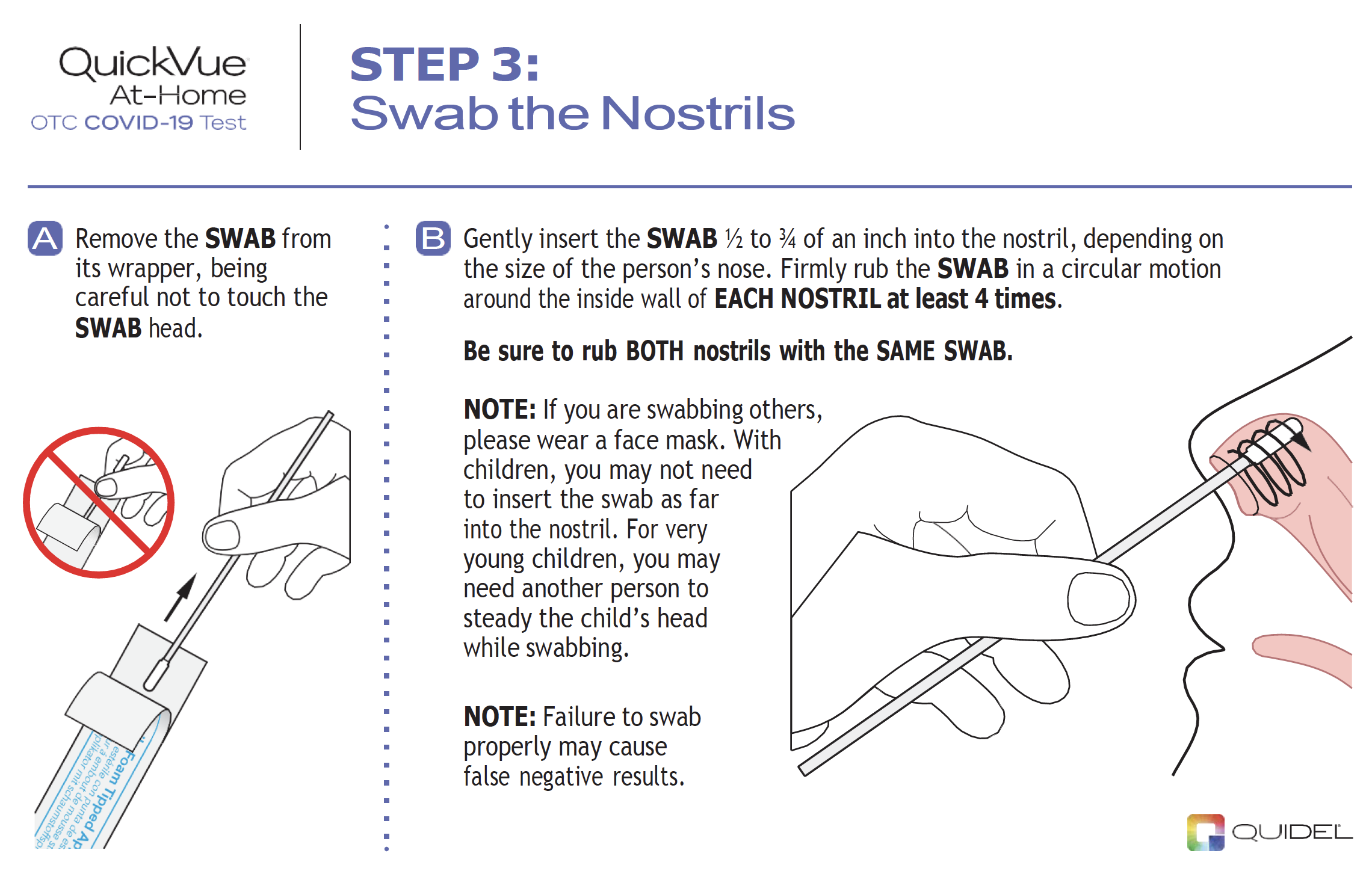 |
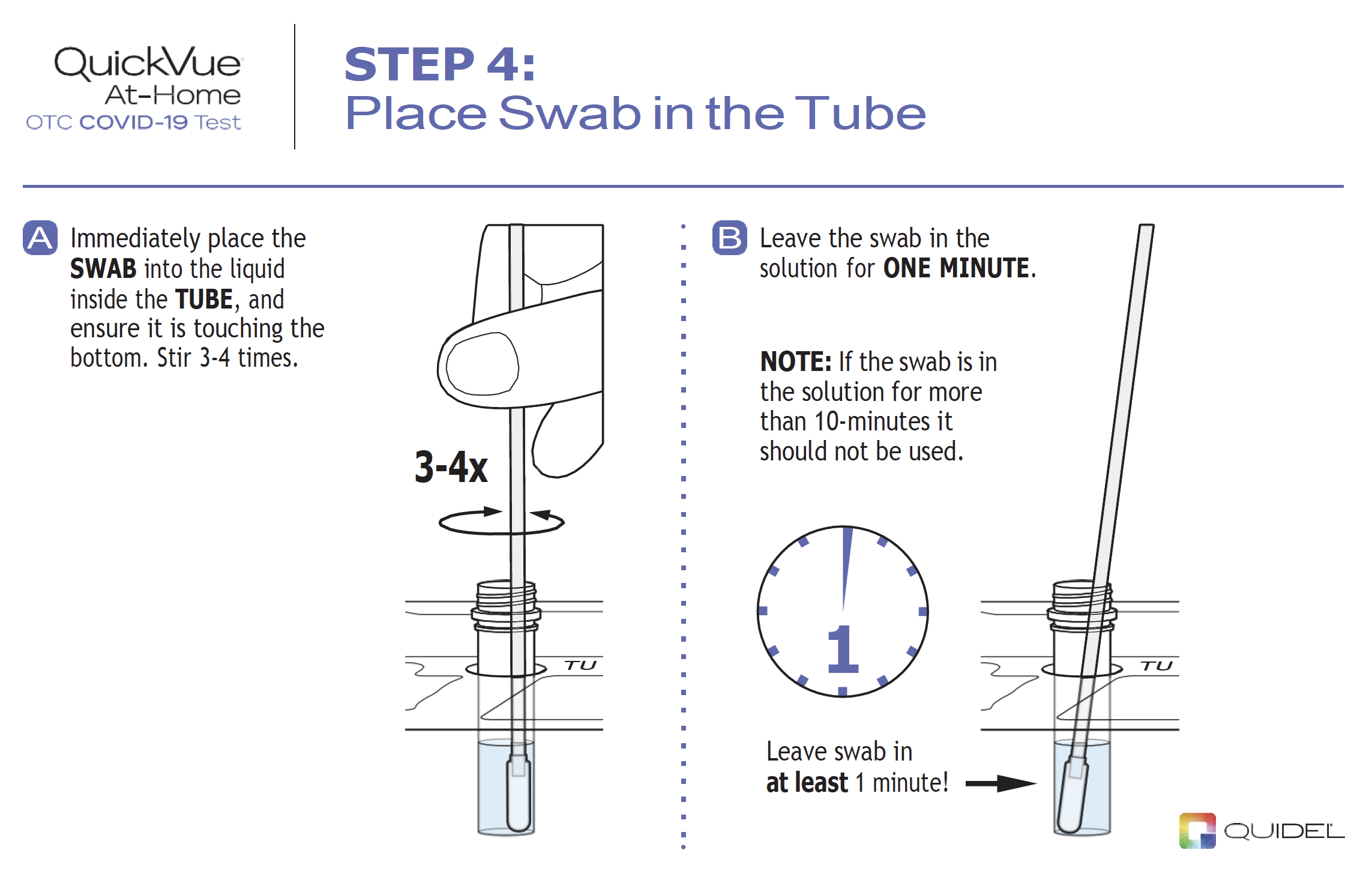 |
 |
 |
 |
 |
 |
 |
 |
 |
 |