ACON 5 Panel Drug Screening Tests
(25 Tests)
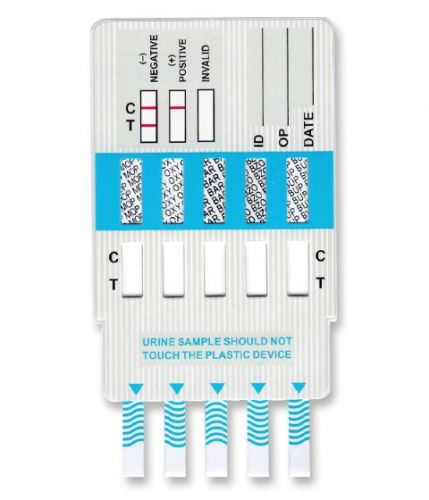
5 Panel Drug Screen, a simple one step dip and read urine test card for detection of Marijuana, Cocaine, Methamphetamine, Opiates and MDMA in urine. FDA Cleared and CLIA Classified as Moderately Complex. (FDA 510K#: K061718)
The ACON 5 Panel Drug Screening Test Card is a Rapid One-step Urine Drug of Abuse Test Kit used to Screen for 5 drugs of abuse and their metabolites at the minimum cutoff sensitivity levels established by the National Institute on Drug Abuse, World Health Organization (WHO) and SAMHSA.
The dip type on-site test kit is simple to use and the results are easily interpreted. The donor voids into the cup and gives the specimen to a collector. The collector inserts the test card into the specimen, removes the test card from the specimen, and places it on a flat surface for processing. The result is ready to read within 5 minutes.
BENEFITS:
- Dip the card in the specimen for 10-15 seconds
- Lie on a flat surface
- Read the results in as little as 5 minutes, but not more than 10 minutes
- The replaceable cap prevents messes
- FDA-Cleared
- Easy-to-use
- Very accurate
- Extremely inexpensive
CPT Code*: 80305 - Drug test(s), presumptive, any number of drug classes, qualitative; any number of devices or procedures, (e.g., immunoassay) capable of being read by direct optical observation only (e.g., dipstick, cups, cards, cartridges) includes sample validation when performed, per date of service (maps to 80300 or G0477).
National Average Reimbursement 2019: $12.60
*All CPT codes are supplied for information purposes only and represent no statement; promise or guarantee by CLIAwaivedTM Inc. that these codes will be appropriate or that reimbursement will be made. It is the responsibility of the service provider to confirm the appropriate coding required by their local Medicare carriers, fiscal intermediaries and commercial payors.